Borne of the careful balance between innovation and regulation, quality medical devices and diagnostics indeed improve the lives of patients. However, quality cannot be tested into the devices.
Instead, it’s up to developers to keep tabs on current quality systems and GxP requirements, and the earlier in the development process these ideals are met, the better the results are for manufacturers and, most importantly, patients.
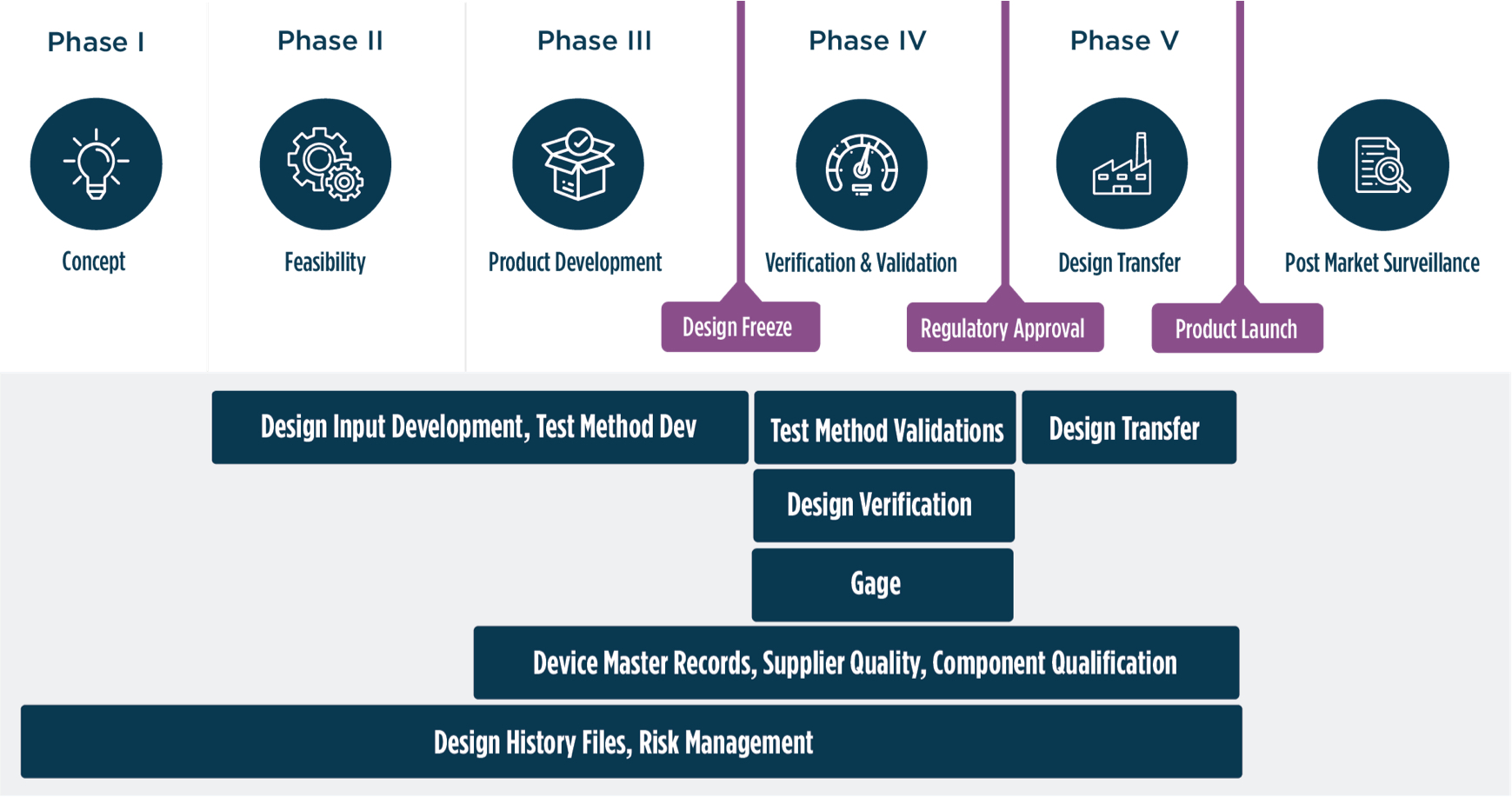
With proactive project management and design controls, we help you start, scale, and sustain® your GxP manufacturing. By balancing project management and design controls, Azzur Group’s guidance helps you meet or beat aggressive plans to enter the market, no matter the class, risk profile, or compliance challenge.