When bringing advanced therapeutics to patients, speed and compliance are critical.
Rapid testing methods from Azzur Labs provide the highest quality and fastest turnaround times into the analytical testing process for complex products.

Rapid testing methods from Azzur Labs provide the highest quality and fastest turnaround times into the analytical testing process for complex products.
Azzur Labs ATP Bioluminescence testing services for rapid sterility provide a non-destructive, no filtration required, method of microbial detection with significantly reduced turnaround times. Requiring a minimal sample size, results for rapid sterility testing are available in as few as 4 days, increasing efficiency by reducing the 14 days of incubation for the traditional USP <71> testing. End user validations for our rapid sterility method include LOD and non-inferiority tests with the compendial method.
Sterility testing is performed in a clean room, and in the case of rapid sterility testing failures, microbial isolates can be immediately identified with our in-house genetic microbial identification capabilities.
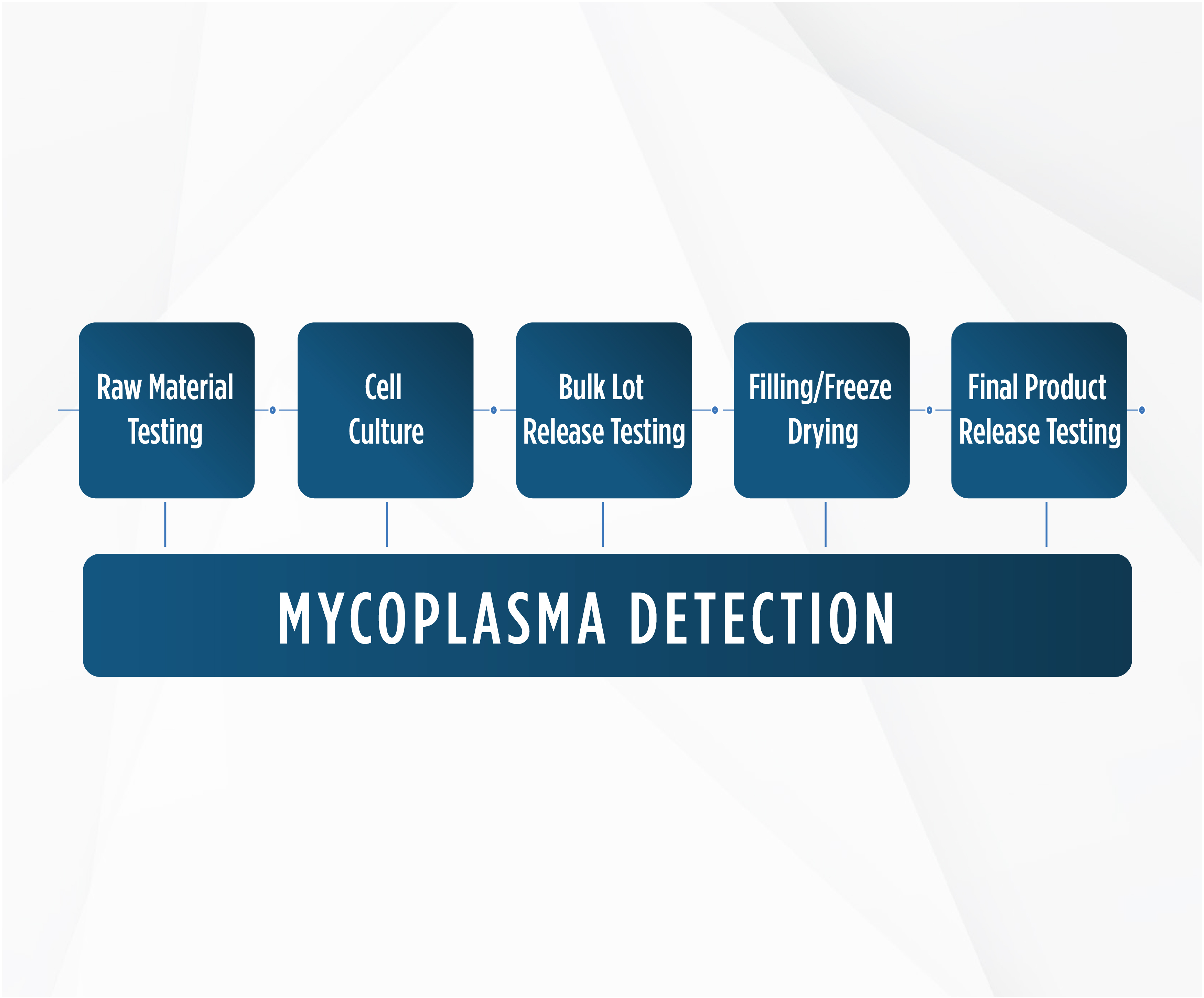
When developing novel therapies, short shelf life and complex CMC designs can present a challenge. Quick turnaround times are essential in order to receive accurate test results and move forward with product development. Cell products or cell substrates used during the advanced therapies manufacturing processes are required to be free of adventitious agents. Rapid Mycoplasma testing services from Azzur Labs provide same-day QPCR mycoplasma detection for use in testing raw materials, cell culture, bulk-lot and final-product release testing, and more. Using matrix effect testing and validation (LOD) for implementation, turnaround time is minimized, and same-day reliable results allow product development to move forward.