Article
Initial Takeaways from FDA Draft Guidance on Selecting a Predicate Device for FDA 510(k) Submissions
September 11, 2023 Mariano A. Mattei
In September 2023, the US Food & Drug Administration (FDA) issued Draft Guidance on Selecting a Predicate Device for FDA 510(k) Submissions.
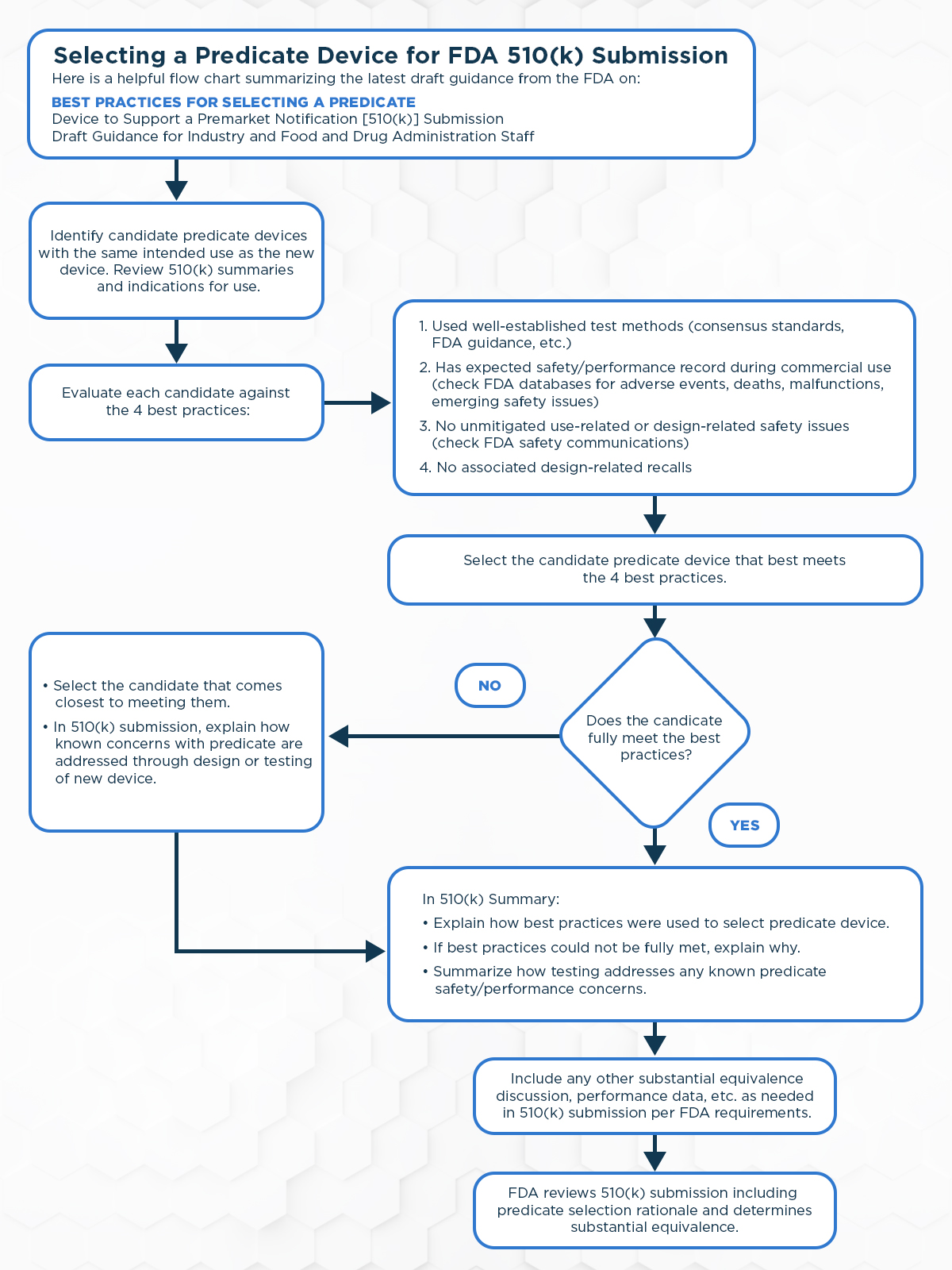
Below are key takeaways from the new draft guidance, as well as a flow chart for easy navigation.
- The guidance provides recommendations for best practices when selecting a predicate device to support a 510(k) premarket submission. The goal is to encourage the use of more modern predicate devices to drive evolution of safer and more effective devices over time.
- Four main best practices are recommended:
- Select a predicate device cleared using well-established methods like FDA guidance, consensus standards, etc. This ensures the latest methods are used.
- Select a predicate with a history of safe and effective performance during commercial use. Review FDA databases for adverse events, deaths, malfunctions, and emerging safety issues.
- Avoid predicates with unmitigated safety issues related to use or design. Review FDA safety communications.
- Avoid predicates that have had a design-related recall, which could indicate fundamental flaws.
- If a predicate fully meeting the best practices is not available, the submitter should explain in the 510(k) how known concerns were addressed through design or testing.
- To improve transparency, submitters should explain in the 510(k) Summary how the best practices were used in predicate selection or why they could not be fully met.
- Examples are provided of predicate selection processes for different device types.
The draft guidance aims to drive selection of more modern predicate devices in 510(k) submissions through recommended best practices, while improving transparency around predicate selection. This is intended to continue advancing safer and more effective devices.
The following flowchart provides a visual representation of the process as outlined in the September 2023 draft guidance:
Related Insights
